A human body is made up of trillions of cells, each carrying the same set of genes but using them in vastly different ways to form functioning organs and tissues. Understanding the roles of each gene—of which there are around 20,000 that encode proteins—remains one of biology’s greatest challenges.
Hertz Fellow Reuben Saunders is up to tackling that challenge. As a graduate student, Saunders developed a method to track the precise effects of thousands of genetic changes across millions of individual cells. His approach uses a version of CRISPR called CRISPR interference (CRISPRi) to silence specific genes without cutting or changing their DNA sequence, and then a combination of sequencing and imaging technologies to study the resulting changes to cells. Moreover, it works on many cells at once—not only in lab dishes, but within the rich complexity of living tissues.
Saunders’s thesis “Pooled genetic screens with rich readouts at scale and in vivo,” describes the method, and its application, and was awarded the 2024 Hertz Thesis Prize from the Hertz Foundation. The Thesis Prize recognizes fellows with exemplary, transformative doctoral theses, and Saunders joins more than 60 fellows who have been previously recognized with the award. Winners are chosen by a vibrant and committed group of volunteers, Hertz Fellows, and non-fellows who serve as Thesis Reviewers.
“We’ve had powerful tools to catalog which genes are active in different cells, but understanding which genes actually control complex tissue behaviors required a new kind of experiment—one that’s quantitative, high-resolution, and scalable in living systems,” said Saunders, who carried out the work under the mentorship of Jonathan Weissman of MIT and the Whitehead Institute for Biomedical Research (previously of University of California, San Francisco).
At the beginning of graduate school, Saunders was interested in studying molecular pathways activated by cells in the face of stress. Weissman’s lab had developed a technology called Perturb-seq in which scientists silence specific genes in different cells and then profile how each change alters which RNA molecules the cell is producing—a proxy for which other genes are being actively used. The technology had previously been used at a small scale in cell cultures, altering only a handful of genes at once.
Saunders and his colleagues had a broader vision: to systematically silence nearly every active gene in human cells and study the effects.
“The early experiments revealed something fundamental: Biology’s complexity isn’t just in having many parts, but in how those parts interact across scales,” said Saunders. “Once I saw that pattern emerging from our data, I knew we had to push beyond cell culture into the messy reality of living tissues.”
Saunders and his collaborators expanded Perturb-seq to a genome-wide level—profiling the consequences of knocking down nearly every expressed gene in a human cell line, across more than 2.5 million cells.
That dataset alone surfaced dozens of new biological insights. For example, Saunders and colleagues discovered how altering genes in the nuclei of a cell—where most DNA is located—led to unexpected changes in the activity of genes in the cell’s energy-generating mitochondria.
“This is what happens when you cast a wide net: You catch things you didn’t know were swimming there. The nuclear-mitochondrial crosstalk emerged from the data like a hidden conversation between two cellular compartments we usually study in isolation,” he said.
But Saunders’s most ambitious leap came in bringing these techniques into live mouse organs. He developed a “genetic mosaic” liver model in which different genes are randomly switched off in some liver cells, while leaving other parts of the liver normal. That provided a well-controlled experiment as to the effect of each gene within a living animal. By studying the resulting livers with a combination of techniques, his team was able to observe how each gene altered cellular physiology. In one striking result, the group found that multiple different gene perturbations caused fat buildup in liver cells—mimicking early fatty liver disease—but through completely distinct molecular mechanisms.
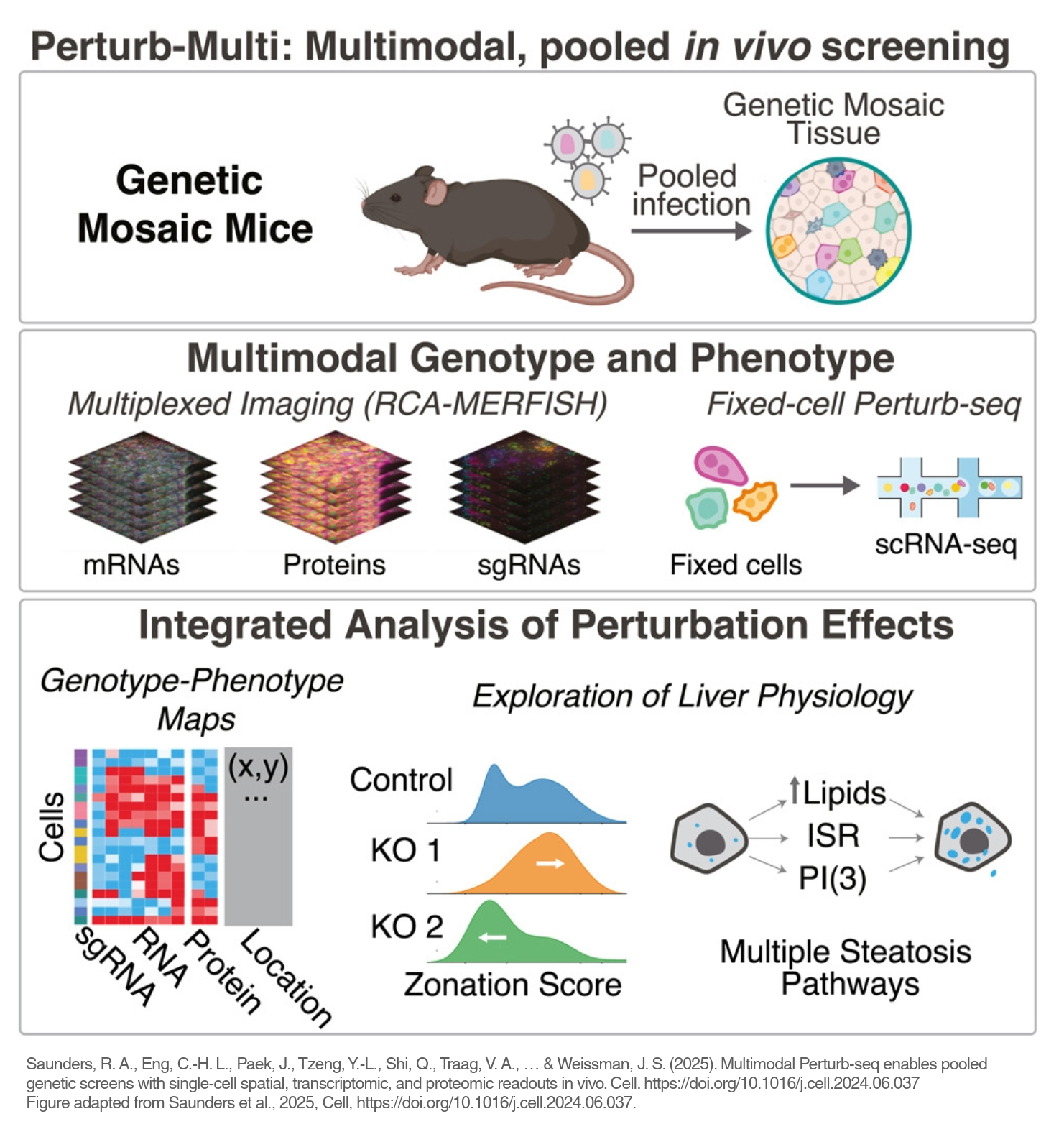
“Those kinds of findings just wouldn’t be possible in traditional animal models, where you can only study one gene at a time,” Saunders said. “By doing these pooled, high-content experiments in vivo, we can start to see the true complexity of how gene function plays out in real physiology.”
Saunders carried out the work not only with members of Weissman’s lab group, but also in collaboration with Xiaowei Zhuang at Harvard and with Will Allen, a Hertz Fellow whom he met through the Hertz community. That connection, Saunders said, was pivotal.
Now a Junior Fellow in the Harvard Society of Fellows, Saunders is continuing to expand the reach of these methods, optimizing their use in organs other than the liver and integrating new types of readouts beyond RNA and imaging—such as epigenetic markers. These tools could ultimately transform how scientists link genes to disease, paving the way for new drugs and therapies.
“The Hertz Fellowship really shaped the course of my research,” he said. “It connected me to collaborators who helped push this work into animal models and imaging—directions I hadn’t originally planned to go.”


